Fabrication of polypropylene meshes deposited with nanofibrous membranes
Polyurethane (PU, YR-80P, Yantai Wanhua Polyurethane Co., Ltd., Yantai, China) or gelatin (Gel, V9000863, Sigma-Aldrich, Waltham, MA, USA) was dissolved in hexafluoroisopropanol to prepare 10% (g/mL) solutions. The two solutions were separately loaded into 5 mL syringes connected to the coaxial needle. The PU solution was used as the core component and Gel solution was the shell. The distance between the needle and the receiving plate was 15 cm, and the rotating speed of the receiver was 150 r/min. The voltage and flow rate of the two solutions were fixed at 20 kV and 0.0020 mm/s, respectively. Coaxial electrospinning was performed at room temperature with humidity of 50%. The collected nanofibrous membrane was vacuum dried for 48 h followed by heating at 120 ℃ for 4 h. The resulting nanofibrous membrane (NM) was attached to both sides of commercial polypropylene mesh (PP, Gynemesh™ TiLOOP, Ethicon, USA) and named as PP + NM. The PP meshes and PP + NM meshes were sterilized with ethylene oxide for subsequent assays.
Morphology characterization: The morphology of the PP mesh was observed by optical microscopy. The morphology of the nanofiber membrane was examined by scanning electron microscopy (SEM, ZEISS Gemini 300, Zeiss, Germany). The surface morphology of the material was examined using an environmental scanning electron microscope (ESEM, FEI, Netherlands, Quanta 200 F) under a vacuum of 130 Pa and at an accelerating voltage of 20 kV. The distribution of nanofibers diameter was calculated by randomly counting and measuring 100 nanofibers in SEM images using the Image J software (National Institute of Health (NIH), Bethesda, MD, USA). The core-shell structure of the nanofibers was observed by transmission electron microscopy (TEM, HT7700, Hitachi, Ibaraki, Japan).
Water contact angle essay and uniaxial tensile test
The water contact angle of the NM was measured by the sessile drop method using a contact angle meter (DAS100, Kruss Scientific, Germany). A volume of 3 μL distilled water was gently dispensed onto the surface of NM to measure the contact angle. Three replicates were performed.
The elastic modulus of NM was measured using an electronic universal testing machine (SLBL, Shimadzu, Japan). The NM was cut into 100 × 15 mm rectangular test pieces and mechanically fractured at a 5 mm/min stretching rate. The elastic, strain, and force were recorded. Three replicates were performed.
Primary cell culture and animal experiments
This study has adhered to the laboratory animal welfare regulations and was approved by the ethics committee of Peking Union Medical College Hospital (no. JS-2240, 25/02/2020). Primary fibroblasts were derived from anterior vaginal wall tissues from patients with stage III or IV anterior vaginal wall prolapse according to the Pelvic Organ Prolapse Quantification (POP-Q). Vaginal wall tissues were collected from patients who underwent surgery for POP at Peking Union Medical College Hospital (PUMCH). Written informed consent was obtained from the participants. Briefly, specimens were cut into pieces smaller than 2 mm and then digested with 10 mg/ml collagenase type I (Sigma, St. Louis, MO, USA) at 37 °C for 2 h. The digestion mixture was filtered through a filter screen with 75-μm pore size and then centrifuged at 1000 rcf for 5 min. The cells at the bottom of the centrifuge tube were mainly fibroblasts. The cells were suspended in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Carlsbad, CA, USA) with 10% fetal bovine serum (FBS; Gibco) and cultured at 37 °C with 5% CO2. Cells at passage 3–7 were used in this study. PP + NM meshes or PP meshes were placed at the bottom of the 96-well or 6-well plates for in vitro assays. Each experiment utilized fibroblasts from three distinct patients as a biological replicate.
This study was in compliance with the NIH Guide for Care and Use of Laboratory Animals. Twenty-four 12-week-old female Sprague-Dawley rats (Charles River, Beijing, China) aged weighing 258 ± 14 g were used in this study. Rats were raised at a temperature of 20–22 ℃, a relative humidity 50–70%, and a 12-hour day/night cycle. Food and water were supplied ad libitum. Our research group previously developed a POP rat model, which was employed for in vivo assays in this study [19]. Briefly, all the rats initially underwent ovariectomy (OVX) in the beginning. Two weeks after OVX, simulated vaginal delivery (SVD) was performed using a size 12 Foley catheter. A balloon injected with 2.5 ml 0.9% saline solution was inserted into the rat vagina and kept in for 4 h.
Implantation of the PP mesh or PP + NM mesh was performed two weeks after SVD. In a slightly moist, yet non-dripping state, the NM can adhere tightly to the surface of the PP mesh. The adhesion is sufficient to ensure successful completion of the surgical implantation procedures. In the experiment, the PP + NM mesh was gently wrapped with sterile gauze in a moist state prior to implantation surgery. During surgery, the PP/PP + NM mesh was sutured to the surrounding tissue for fixation. The incision site located at the midpoint of the lateral wall of the rat vagina was selected, as depicted in Fig. 1A. A 2 cm incision was made in the mucosa layer of the vaginal wall, and subsequently, either PP mesh or PP + NM mesh was placed in the sub-mucous layer. The mesh was sutured intermittently to secure it between the smooth muscle layer and connective tissue, followed by closure of the wound (Fig. 1B). Each group had 6 biological replicates at each time point.
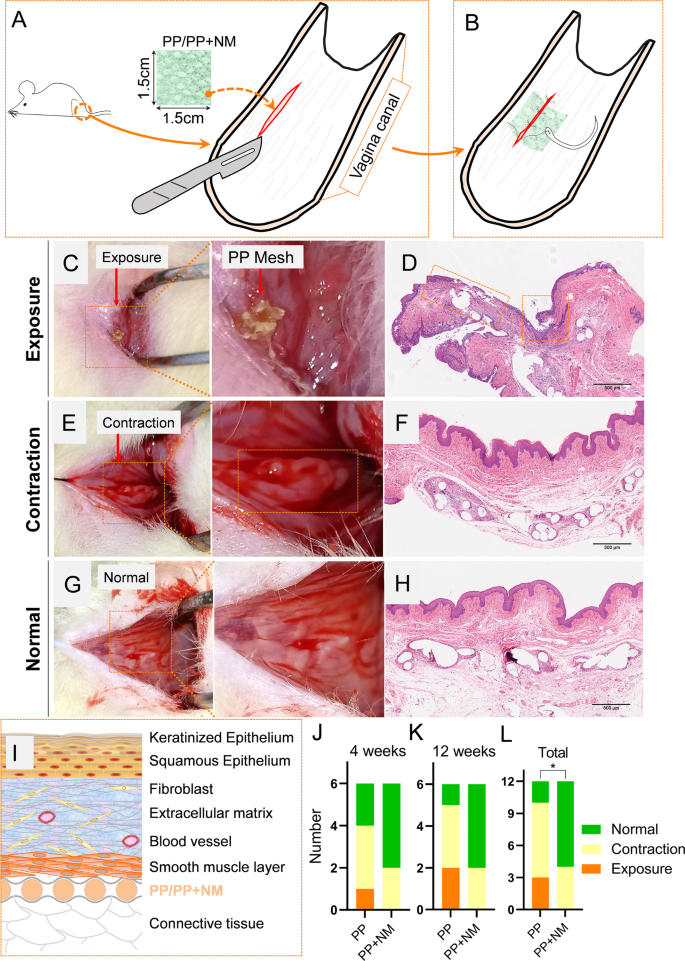
In vivo evaluation of the modification effects on the complications of polypropylene (PP) mesh. Schematic diagram of the surgery process of implanting the mesh in rats (A–B). Representative images depicting the exposure, contraction, and normal states in a macroscopic view and hematoxylin-eosin staining (C–H). Schematic diagram of the structure of the vaginal wall and the position of the implanted mesh in rats (I). The number of exposures, contraction, and normal status at 4 weeks and 12 weeks (J–K). The combined data from two time points (L)
Cell counting kit-8 assays
Primary fibroblast cells (4 × 103) were seeded into a 96-well plate (Corning) with trimmed PP mesh or PP + NM mesh in the wells. When the cell fusion reaches approximately 75%, cell counting kit 8 regent (CCK-8, Dojindo; Kumamoto, Japan) diluted with culture medium in 1:10 were added to each well and incubating for 45 min. Absorbance was measured at 450 nm using the Thermo Fisher Varioskan Flash (Thermo Fisher, CA, USA) microplate reader.
Immunofluorescent staining for F-actin
An actin-tracker red-555 kit (Beyotime, Shanghai, China) was used to visualize the filamentous actin (F-actin) of fibroblasts. Briefly, primary fibroblast cells on PP + NM were fixed using 4% paraformaldehyde for 15 min and washed with phosphate-buffered saline (PBS) containing 0.1% Triton X-100 three times. The working solution was then added and incubated for 60 min. After washing with PBS, cells were incubated with a 5 μg/mL DAPI solution for 5 min and washed with PBS three times. Cells were observed and photographed immediately using a fluorescence microscope (Nikon, Tokyo, Japan).
Hematoxylin-eosin (HE) staining
Primary fibroblasts cultured above the PP + NM meshes for 48 h or the PP + MN implanted POP rat vaginal wall were fixed using 4% paraformaldehyde for 24–48 h. The dehydrated samples were embedded in paraffin, and then cut into sections (transection, 4 μm). After dewaxing, the slides were ready for HE staining. The slices were stained with hematoxylin for 5 min and rinsed with distilled water. After differentiation with alcohol hydrochloride for a short time, lithium carbonate was added for 2 min and then washed with distilled water. The slides were then stained with eosin solution for 50 s and rinsed with distilled water. After dehydration and treatment with xylene, the slides were covered with liquid mountant and coverslip. The slides were observed and photographed using a optical microscope (Nikon).
RNA-sequencing
Primary fibroblasts were seeded into a 24-well plate (Corning) with trimmed PP mesh or PP + NM mesh in the wells. The cells were cultured for 48 h. After the cell culture medium was removed, the cells were washed with PBS at 4℃ and the lysis buffer was then directly added. Isolation of total RNA was performed using the FastPure Cell/Tissue Total RNA Isolation Kit V2 (Vazyme Biotech, Nanjing China) according to the manufacturer’s instructions. For RNA-sequencing, the NEBNext Ultra Directional RNA Library Prep Kit was used to prepare the library and the library was sequenced using the HiSeq PE150 platform (Illumina, San Diego, USA). GSEA analysis was performed using (last accessed on 10 August 2024), an online platform for data analysis and visualization [20]. A P value under 0.05 and absolute log2 value of a fold-change greater than 2 were considered as statistically significant.
Picro-Sirius red staining
Slides were prepared as described above and were stained using Picro-Sirius red F3BA (Sigma, St. Louis, MO, USA) according to the manufacturer’s instructions. The slides were observed and photographed using a polarization microscope (Nikon). Images were analyzed using Image-Pro Plus Version 6.0 software (Media Cybernetics, Silver Spring, USA). The red and yellow areas were identified as type I collagen and the green area was identified as type III collagen. The ratios of collagen I to collagen III were calculated for comparison among groups. They were observed and photographed using a polarization microscope (Nikon).
Immunohistochemistry
Slides were prepared as described above. The antigens were retrieved using citrate buffer and blocked by bovine serum albumin (Sigma). The slides were then incubated with specific antibodies at 4 ℃ for at least 12 h. The antibodies used in this study included ACTA2 (1:200, ABclonal; Wuhan China), matrix metalloproteinase 2 (MMP2) (1:100, ABclonal), MMP9 (1:100, ABclonal), tissue inhibitor of metalloproteinase-1 (TIMP1, 1:200, Invitrogen; Carlsbad, CA, USA), TIMP2 (1:100, ABclonal), F4/80 (1:4000, Proteintech, Wuhan, China), and TNF-α (1:1000, Proteintech). Horseradish peroxidase conjugated secondary antibodies were then incubated (Solarbio, Beijing, China) at room temperature for 30 min. Then, a DAB substrate kit (Solarbio) was used to visualize the sections. We used an optical microscope (Nikon) for photographing and the Image-Pro Plus Version 6.0 software (Media Cybernetics) for quantitative analysis of the images. We compared the percentage of positive area between groups. Microvascular density was assessed through regular immunofluorescent staining utilizing von Willebrand factor (vWF, 1:1000, Proteintech, Wuhan, China).
Western blot assays
Rat vaginal wall tissues at the mesh implantation site were collected and lysed in RIPA lysis buffer supplemented with protease and phosphatase inhibitors (Beyotime) at 4 ℃. The lysates were then centrifuged at 12,500 g for 15 min. Proteins in the supernatants were quantified by Bradford protein assay kit (Beyotime). Proteins were separated by 10% SDS-polyacrylamide gel (Invitrogen) and were transferred onto polyvinylidene difluoride (PVDF, Invitrogen) membranes by an iBlot® Gel Transfer Device (Invitrogen). Antigens were blocked by a blocking buffer for western blot (Beyotime). The membranes were then incubated on shaking table at 4 ℃ for at least 12 h with primary andibodies, including GAPDH (1:1000, Cell Signaling Technology, CST, Danvers, MA, USA), alpha smooth muscle actin (ACTA2, 1:1000, ABclonal), COL1 (1:2000, Proteintech), collagen-3 (COL3, 1:1000, Proteintech), TIMP1 (1:1000; ABclonal), TIMP2 (1:1000; ABclonal), MMP2 (1:1000; Abcam; Cambridge, UK), MMP9 (1:1000; Abcam), transforming growth factor-β1 (TGF-β1, 1:1000, ABclonal), P-Smad3 (1:1000, ABclonal), Smad3 (1:4000, Proteintech), P-p38 (1:1000, CST), p38(1:1000, CST), P-ERK1/2 (1:2000, CST), ERK1/2 (1:1000, CST), and Tubulin (1:1000, Solarbio). Horseradish peroxidase conjugated secondary antibodies (Solarbio) were then incubated at room temperature for 1 h. The immunoblots were visualized using an enhanced chemiluminescence (ECL) kit and photographed by gel imaging system (Tanon, Shanghai, China). Relative density of the immunoblot fragments was quantified using the Image J software.
Statistical methods
The software GraphPad Prism 9 software (GraphPad Software, Inc., USA) was used for statistical analyses and graphing. Descriptive data are presented as the means and standard deviations (SDs). Two-tailed Student’s t tests were used to compare mean differences between two groups. Fisher’s exact test was used to compare the incidence of mesh complications between groups. Statistical significance was defined as P < 0.05.



