Synthesis and characterization of FeS2@COF-HA/AIPH
The synthesis process of FeS2@COF-HA NPs is shown in Fig. 1a. Meanwhile, effective preparation of the FeS2, COF, and surface functionalization were confirmed by Transmission electron microscopy (TEM) and scanning electron microscopy (SEM). As depicted in Fig. 1b, the FeS2 NPs had a uniform spherical shape with a mean diameter of approximately 8 nm. The FeS2 NPs incorporating in COF NPs via a general encapsulation method. TEM images (Fig. 1c, d) and SEM images (Fig. S1 and S2) exhibited that both the synthesized TAPB-DMTP-COF and FeS2@COF were uniformly spherical with an average size of 170 nm, demonstrating that FeS2 NPs had no effect the growth of COF NPs. Dynamic light scattering (DLS) analysis also validated that the particle size of the prepared FeS2@COF was mostly concentrated at 180 nm (Fig. 1e). Notably, the sizes of FeS2@COF measured by TEM were smaller than that measured by DLS, which was attributed to the hydration effect. To further substantiate the successful synthesis of FeS2@COF NPs via FeS2 encapsulation, the high-angle annular dark-field scanning transmission electron microscopy (HADDF-STEM) image and corresponding energy dispersive spectrometer (EDS) elemental mapping images manifested that the Fe and S element were obviously concentrated inside the COF, which indicated the successful formation of FeS2@COF NPs (Fig. 1f). Specifically, the C, N, and O element were derived from COF, while the Fe and S element corresponded to FeS2.
UV-vis-NIR spectrum of FeS2 NPs showed broad absorbance in the NIR region, demonstrating its superior photothermal effects (Fig. 1g). The structure of the nanocomposites was further characterized by XRD. Following the encapsulation of FeS2 NPs, the XRD pattern of FeS2@COF displayed the distinct diffraction peaks of both FeS2 and TAPB-DMTP-COF. This confirms the successful preparation of the FeS2@COF NPs and demonstrates that the incorporation of FeS2 NPs does not alter the structure of TAPB-DMTP-COF (Fig. 1h). Meanwhile, the results of X-ray photoelectron spectroscopy (XPS) further indicated that there was a significant Fe and S signal peak, demonstrating the presence of FeS2 NPs (Fig. 1i). To enhance the biocompatibility and tumor cell targeting ability, the surface of FeS2@COF NPs was further coated with FA modification. The alteration of surface zeta potentials of nanocomposites proved the successful modification in each step (Fig. 1j). In addition, the particle size of FeS2@COF-HA dispersed in three types of physiologically relevant media, including water, PBS and cell culture medium, was monitored over 7 days using DLS and TEM. As shown in Fig. S3, the particle size and morphology of FeS2@COF-HA did not significantly change, demonstrating superior colloidal stability in physiologically relevant environments.
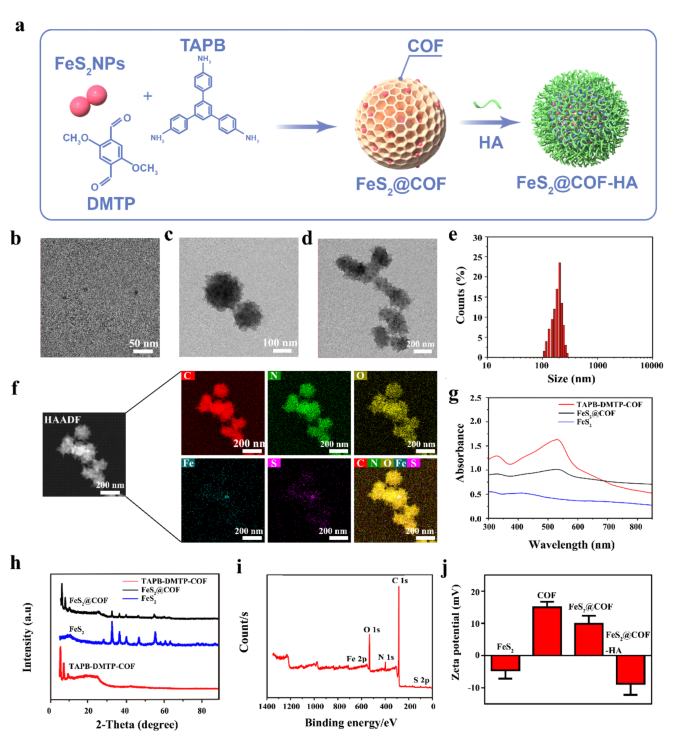
Synthesis and characterization of FeS2@COF-HA. (a) Schematic diagram showing the successive preparation process of FeS2@COF-HA NPs. (b-d) TEM images of FeS2, TAPB-DMTP-COF and FeS2@COF. (e) Size distribution of the as-prepared FeS2@COF. (f) HAADF-TEM image and elemental mapping results of FeS2@COF. (g, h) UV-vis spectra and XRD patterns of TAPB-DMTP-COF, FeS2, and FeS2@COF. (i) The XPS full survey of FeS2@COF. (j) Zeta potentials of FeS2, TAPB-DMTP-COF, FeS2@COF and FeS2@COF-HA
In vitro photothermal performance
After proving the successful synthesis of FeS2@COF-HA, we further investigated the photothermal effects of FeS2@COF-HA nanocomposites by recording the temperature change of FeS2@COF-HA dispersion at different concentrations upon irradiation of 808 nm laser (1.0 W/cm2, 10 min). Noteworthy, a significant temperature elevation was observed with an increased concentration of FeS2@COF-HA dispersion (Fig. 2a and b), substantiating that the photothermal effect of FeS2@COF-HA was dose-dependent. Meanwhile, the photothermal heating curves of FeS2@COF-HA solutions exhibited a laser power-dependent photothermal performance (Fig. 2c and S4). Beyond that, the temperature variation of different samples during 808 nm irradiation were recorded (Fig. 2d). Specifically, the temperature is positively associated with the irradiation time in the FeS2@COF-HA and FeS2 group compared to the water and COF group (Fig. 2e), which demonstrated that the photothermal effects of the FeS2@COF-HA originated from the strong NIR absorption of FeS2 NPs. Moreover, the photothermal conversion efficiency (η) of FeS2@COF-HA NPs was calculated to be 28.7% according to the previous method [49] (Fig. 2f and g), which suggested that FeS2@COF-HA was capable of converting laser energy into local hyperthermia. Simultaneously, the photothermal performance of FeS2@COF-HA still did not deteriorate after 5 irradiation/cooling cycles, suggesting the excellent photothermal stability (Fig. 2h). Thus, these results validated that FeS2@COF-HA could be used as a potent photothermal agent for NIR-triggered alkyl radical generation of AIPH.
AIPH loading and pH/NIR triggered release of the agents
The potential of FeS2@COF-HA as drug delivery system to encapsulate the alkyl radical initiator (AIPH) was further investigated. Based on spectrophotometry, the drug loading content (LC) was measured to be 54.3 ± 2.1% from the stand curve of AIPH (Fig. S5), originating from the large pore surface area of FeS2@COF. To confirm the controllability of dual intelligent stimuli responsive “gatekeepers”, under low pH stimulation and NIR laser irradiation, the release profiles of AIPH from FeS2@COF-HA were detected. Notably, the HA coating could be dissociated for the release of Fe2+ and AIPH in a pH-responsive and NIR-enhanced manner. As illustrated in Fig. 2i, negligible drug release was observed at pH = 7.4 with NIR irradiation or without NIR irradiation. On the contrary, the AIPH release at pH 5.0 reached 39.6% after 24 h and further significantly increased to 57.2% upon NIR laser irradiation. In addition, we investigated the release behavior of the Fe2+ from the nanoplatform. As can be seen from Fig. S6, under the neutral condition of pH 7.4, the release of Fe2+ was insignificant. Nevertheless, once the increase of H⁺ occurred (pH 5.0), an explosive release of Fe2+ was noted after the same incubation time. Specially, it was noticed that the release rate of the Fe2+ could be remarkably enhanced through the involvement of NIR irradiation. Therefore, the in vitro drug release results provided clear evidence that FeS2@COF-HA possess the ability to respond to acidic TME condition and NIR laser irradiation, which could effectively prevent normal cell damage from the instantaneous generation of excessive free radical and allow for an on-demand controlled therapeutic agents release for continuous tumor therapy.
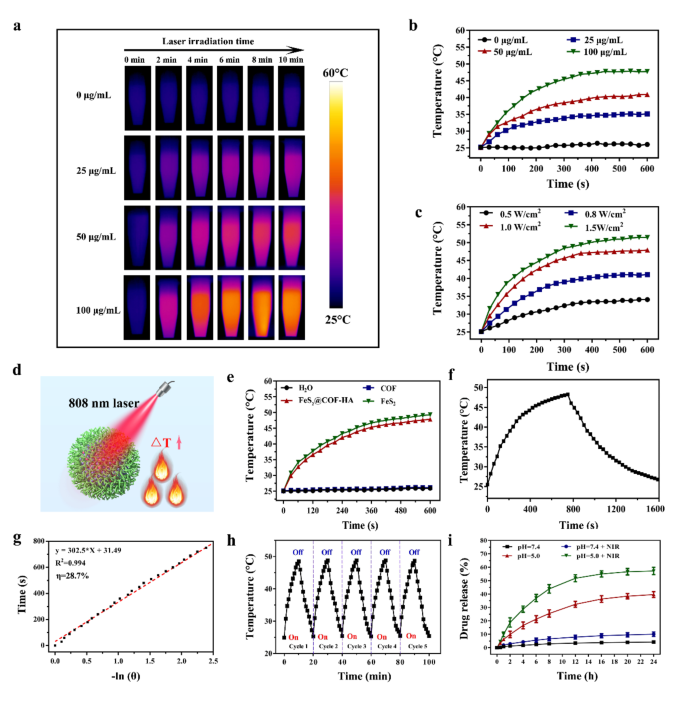
(a, b) Representative photothermal images and temperature variation curves of different concentrations of FeS2@COF-HA solution upon irradiation of 808 nm laser (1.0 W/cm2, 10 min). (c) Temperature variation curves of FeS2@COF-HA solution under different power densities irradiation. (d) Schematic illustration of the photothermal effect of FeS2@COF-HA under NIR irradiation. (e) Temperature elevation of various samples (100 µg/mL) upon irradiation of 808 nm laser (1.0 W/cm2, 10 min). (f) Temperature changes of FeS2@COF-HA solution with irradiation for 750 s, and then the laser was turned off. (g) Linear time data vs. -ln(θ) gained from the cooling period of FeS2@COF-HA. (h) Temperature changes of FeS2@COF under 808 nm laser irradiation for five cycles. (i) AIPH release behavior from FeS2@COF-HA/AIPH under different conditions
Free radical generation capacity
Inspired by the superior photothermal conversion effects of FeS2@COF-HA NPs, we further investigated the alkyl free radical production ability of FeS2@COF-HA/AIPH upon laser irradiation by utilizing 2,2′-azobis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) as a chemical probe to capture free radicals under different conditions (Fig. 3a and b). Observed from Fig. 3c, only small amount of alkyl radical was monitored when FeS2@COF-HA/AIPH placed at 37 °C, while a lot of alkyl radicals were generated continuously at 44 °C, demonstrating that the high temperature is beneficial for the formation of alkyl radicals. Meanwhile, laser-activated alkyl radical production by FeS2@COF-HA/AIPH was further detected (Fig. 3d). Typically, the ability to generate alkyl radicals could be effectively strengthened with the prolonged irradiation time, suggesting a strong correlation between the generation of alkyl radicals and the duration of laser exposure. However, alkyl radicals were not detected in the AIPH and FeS2@COF-HA group under the same conditions (Fig. 3e). Furthermore, the absorption intensity of ABTS+• exhibited an approximate linear relationship with the prolongation of irradiation time (less than 5 min). However, the absorbance stabilized in the subsequent 5 min, which is attributed to the complete photo-induced degradation of AIPH (Fig. 3f). These results indicated a promising potential for utilizing FeS2@COF-HA/AIPH in TDT, as it was capable of generating alkyl radical under the condition of laser-triggered hyperthermia.
Cellular uptake and intracellular ·OH detection
During the process of cancer therapy, delivering drugs effectively to tumor cells remains a formidable challenge [51]. Thus, prior to investigating the in vivo therapeutic outcomes, the tumor-targeting ability of FeS2@COF-HA was first evaluated. Obviously, it could be seen that the strong red fluorescence signal was found in the FeS2@COF-HA treated cells group (Fig. S6), whereas no significant fluorescent signal was noted in FeS2@COF group. Thus, these findings indicated that the functionalization of HA on the surface of FeS2@COF-HA could remarkably enhanced the overall accumulation of nanocomposites within the targeted tumor cells, enabling the FeS2@COF-HA suitable candidates for specific tumor therapy.
Temperature is an important factor that impacts the rate of Fenton-like reaction, where a rising local temperature can remarkably enhance the CDT efficiency [52, 53]. Hence, ·OH radical production activated by FeS2@COF-HA in the presence or absence NIR laser irradiation are comprehensively detected by utilizing 3,3′,5,5′-Tetramethylbenzidine dihydrochloride (TMB) (Fig. 3b). Notably, FeS2@COF-HA exerted a strong ·OH signal in the presence of H2O2, while the other groups exhibited no obvious change, indicating that the ·OH radical generation was generated based on the reaction of FeS2@COF-HA and H2O2 (Fig. 3g). In addition, as the NIR irradiation added to the FeS2@COF-HA group, the characteristic absorption peaks of TMB at 665 m, were remarkably enhanced, which demonstrated that the locally high temperatures produced by NIR laser irradiation remarkably enhanced the catalytic ability of the Fenton reaction. In addition, electron spin resonance (ESR) spectroscopy was performed to detect the generation of •OH by using 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) as the trapping agent. In comparison to the FeS2@COF-HA and AIPH + H2O2 groups, a group of typical radical peaks was observed in the FeS2@COF-HA + H2O2 group, while the typical peak intensity was significantly elevated after irradiating with the 808 nm laser irradiation (Fig. Sx), confirming that the thermal effect of laser irradiation for the promotion for CDT. For more specific analysis, methylene blue (MB) was explored to detect the generation of ·OH radicals under the condition of various pH values (pH = 5.5, 6.5, and 7.4). As displayed in Fig. 3h, the absorption peak at 660 nm was significantly lower than that at high pH values, suggesting that a low pH value could promote the efficiency of the Fenton-like effect. Taken together, all the above results revealed that the Fe2+ could effectively trigger the Fenton reaction of FeS2@COF-HA to produce ·OH radicals.
To further confirm the generation of ROS within cells, the fluorescent probe 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) was employed for detect the total generation of intracellular ROS (Fig. S7). By contrast, a strong green fluorescence signals were noted in the FeS2@COF-HA and FeS2@COF-HA + laser groups, reflecting that the endogenous H2O2 is converted to harmful ·OH radicals via Fe2+-mediated Fenton-like reactions. More importantly, after MNNG/HOS cells were treated with FeS2@COF-HA/AIPH and irradiated with 808 nm laser under both normoxia and hypoxia conditions, the strongest green fluorescence can be observed, demonstrating the highest intracellular ROS level. This improvement was attributed to the generation of both ·OH radicals and alkyl radicals induced by hyperthermia. Furthermore, these findings also demonstrated that the significant generation of intracellular ROS by FeS2@COF-HA/AIPH is independent of levels of oxygenation, holding great potential for enhanced antitumor effects.
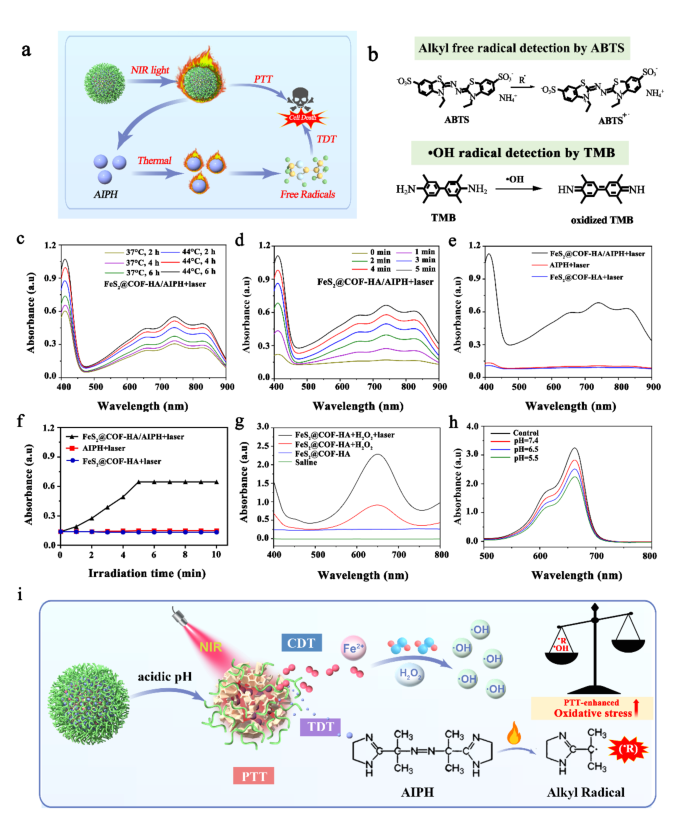
(a) Schematic illustration of the designed experiment for alkyl radical generation under the NIR laser irradiation. (b) The reactions of free radicals captured by ABTS and TMB. (c, d) Generation of free radicals at different temperatures and time points. (e) Generation of free radicals in different groups. (f) The absorbance changes of ABTS in different groups following irradiating a 808 nm laser for 10 min. (g) ·OH radical generation in different groups. (h) The absorbance of MB with the addition of FeS2@COF-HA at different pH values. (i) Schematic illustration of the TME-responsive degradation of FeS2@COF-HA/AIPH and the subsequent PTT-enhanced CDT and NIR-triggered TDT
In vitro anti-tumor effect
Encouraged by the controlled drug release behavior, excellent photothermal effect and potential of booming ·OH generation ability, we further explored the combinational therapeutic outcomes of FeS2@COF-HA/AIPH in vitro (Fig. 4a). Obviously, the results of calcein-AM/PI staining verified that FeS2@COF-HA/AIPH + laser caused apparent cell death to MNNG/HOS cells when compared to other groups (Fig. 4b), further demonstrating the desirable combined PTT/CDT/TDT effects upon NIR laser irradiation. Moreover, the cytotoxicity of FeS2@COF-HA/AIPH on MNNG/HOS cells was further assessed by cell-counting kit-8 (CCK-8) assay. As illustrated in Fig. 4c, no evident toxicity was noted with or without laser irradiation when AIPH was used. However, after treatment with FeS2@COF-HA, cell viability was moderately decreased due to the CDT effect. Meanwhile, 808 nm laser irradiation further enhanced the detrimental effect on tumor cells, benefitting from the improved therapeutic modality synergized by PTT. As expected, the most substantial detrimental effect on cell viability was observed following the administration of FeS2@COF-HA/AIPH + laser group, resulting in a reduction of approximately 10% in cell viability under both normoxia and hypoxia conditions. Additionally, the results of colony formation also exerted that FeS2@COF-HA/AIPH + laser group exhibited the strongest lethality for MNNG/HOS cells under both normoxia and hypoxia conditions (Fig. 4d). The combination index (CI) of PTT/CDT/TDT is calculated to be 0.47, verifying the synergistic effect. Overall, this exceptional cell damage can be ascribed to the combinatorial effect of PTT/CDT/TDT, with treatment efficacy independent of oxygenation level, making it highly favorable for eliminating deep-seated tumors.
Numerous studies have proved that mitochondrial damage was closely associated with ROS-induced oxidative stress [54,55,56], and the mitochondrial membrane potential (MMP) served as an important and representative indicator for assessing mitochondrial function (Fig. 4e). Therefore, 5,5,6,6-tetrachloro-1,1,3,3-tetraethylbenzimidazolocarbocyanine iodide (JC-1) probe was then utilized to investigate the impact of nanoplatform on mitochondrial function. Generally, the JC-1 probe displayed red fluorescence under normal MMP conditions, while under abnormal conditions, it showed green fluorescence. As shown in Fig. 4f, the results exerted that FeS2@COF-HA/AIPH + laser treated group showed the strongest green fluorescence under the condition of normoxia and hypoxia, which indicated that FeS2@COF-HA/AIPH + laser could effectively induce abnormalities in the MMP of MMNHOS cells by generating an excessive generation of ROS [54]. Moreover, the fluorescence intensity ratio of green/red fluorescence in MNNG/HOS cells was remarkably higher than that observed in the other groups following treatment with FeS2@COF-HA/AIPH + laser (Fig. S8), demonstrating that its antitumor effect was more significant. Simultaneously, there was no significant difference in fluorescence intensity ratio between groups (6) and (7), indicating that the therapeutic outcomes of FeS2@COF-HA/AIPH + laser was independent of the local oxygenation level. Additionally, the process of cell apoptosis was further investigated using the Annexin V-FITC/PI staining assay through flow cytometry. In contrast to the other groups, treatment with FeS2@COF-HA/AIPH + laser resulted in the highest rate of apoptosis in both normoxia and hypoxia (Fig. 4g and h), which can be attributed to the outstanding therapeutic outcomes resulting from the synergistic effects of PTT/CDT/TDT.
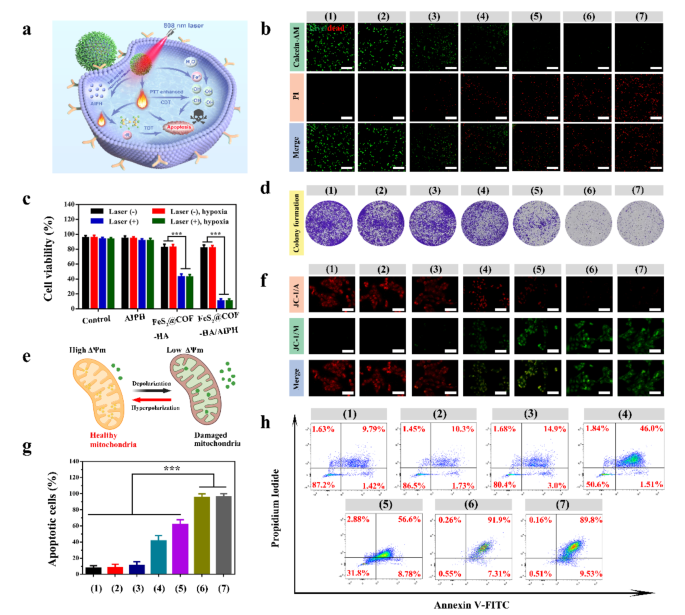
Therapeutic effects of PTT/CDT/TDT in vitro. (a) Schematic illustration to indicate FeS2@COF-HA/AIPH-mediated synergistic therapeutic effects against MNNG/HOS cells. (b) The live/dead staining images of the treated cells in different groups (scale bar: 100 μm). (c) Viability of MNNG/HOS cells receiving different treatments under normoxia and hypoxia conditions. (d) Representative photographs of stained colonies of MNNG/HOS cells treated with different groups. (e) Working principle of JC-1 assay for monitoring MMP change. (f) JC-1 assay for illustrating the depletion of MMP in MNNG/HOS cells treated with corresponding treatments. (g, h) Quantification of apoptotic MNNG/HOS cells and Annexin V-FITC/PI assay after different treatments: (1) control, (2) laser, (3) AIPH + laser, (4) FeS2@COF-HA, (5) FeS2@COF-HA + laser, (6) FeS2@COF-HA/AIPH + laser (normoxia), (7) FeS2@COF-HA/AIPH + laser (hypoxia). All data are presented as means ± standard deviation (n = 3)
Immune activation effect of nanocomposites
Immunotherapy act a key role in inhibiting tumor proliferation, and the effectiveness of immunotherapy correlated significantly with macrophage polarization [57, 58]. Moreover, it is known that intracellular oxidative stress generated by Fe2+ play an important role in the activation of immune response, which could effectively promote the polarization of M1 macrophages [59]. Therefore, we then explored the impact of FeS2@COF-HA on reprogramming M2 macrophages by investigating its influence on macrophage polarization in vitro (Fig. 5a). As illustrated in Fig. 5b-d, FeS2@COF-HA/AIPH + laser group could significantly increase the CD86 expression by 11.5% and 50.1%. Meanwhile, the CD206 expression dramatically decreased from 62.0 to 24.7%. Importantly, immunofluorescence staining was utilized to further investigate the polarization of the RAW 264.7 cell after corresponding treatment. As displayed in Fig. 5e and f, the fluorescent intensity of M1 macrophages (red fluorescence) increased, while M2 macrophages (green fluorescence) decreased following FeS2@COF-HA/AIPH + laser treatment. Collectively, these findings verified that FeS2@COF-HA combined with AIPH encapsulation under laser irradiation could effectively repolarize M2-like macrophages by upregulating M1-related CD86 markers and downregulating M2-related CD206 markers.
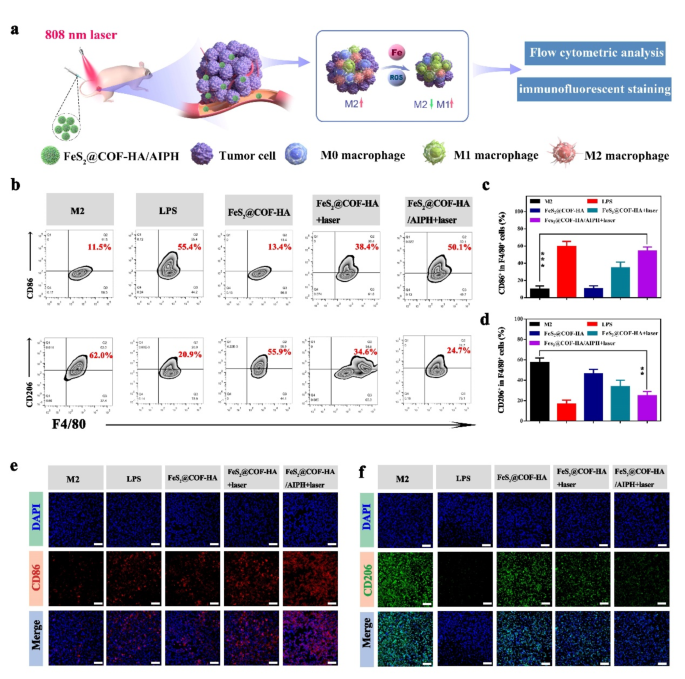
FeS2@COF-HA/AIPH can activate antitumor immunity. (a) Schematic illustration for mechanism of macrophage M1 repolarization. (b) Flow cytometric results of the expression of CD86 and CD206 after corresponding treatments. (c, d) Quantitative analysis of CD86+and CD206+ in flow cytometry. (e, f) Representative immunofluorescent images of the expression of CD86 and CD206 in different groups. (Scale bar: 50 μm)
In vivo biodistribution and photothermal imaging
To further validate the tumor-targeting accumulation of the nanocomposites, the tumor specific imaging and biodistribution were explored by a small animal in vivo fluorescence imaging system. As demonstrated by the in vivo bioimaging, the FeS2@COF-HA group displayed strong fluorescent signals at the tumor site 6 h after intravenous injection (Fig. 6a), whereas the control group only exhibits mild fluorescence signal. Also, the ex vivo imaging also exhibited that FeS2@COF-HA were mostly accumulated in the tumor region (Fig. 6b). Meanwhile, the fluorescence images of the tumor slice further confirmed this conclusion (Fig. 6c). Taken together, these results revealed that FeS2@COF-HA can specifically target into the tumor sites as an ideal nanocarrier for the highly efficient and safe delivery of drugs to tumor site.
Based on the results of above imaging observations, the NIR laser irradiation should be carried out at 6 h post-injection, and this time point was adopted for the subsequent in vivo photothermal therapy experiment. The thermal imaging photos exhibited the temperature of FeS2@COF-HA group increasing obviously, while the temperature of control group exhibited a moderate temperature increase in tumor sites following injection for 6 h under laser irradiation (Fig. 6d and e). These observations suggested that the FeS2@COF-HA possessed superior photothermal property, which could effectively induce local hyperthermia for tumor therapy.
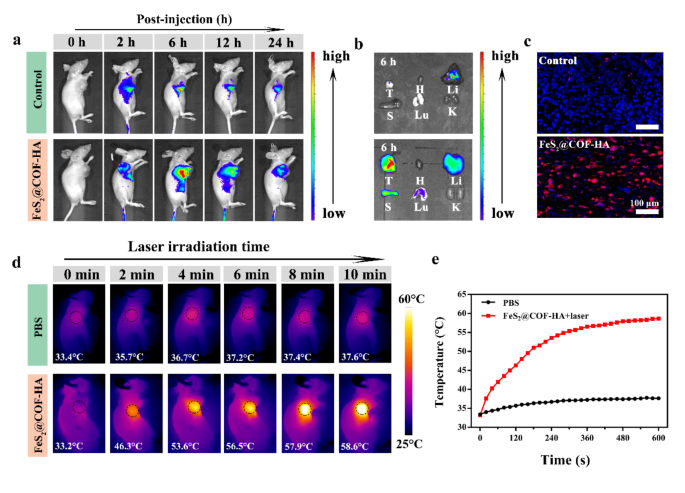
In vivo tumor specific targeting and thermal imaging. (a) Real-time fluorescence images of MNNG/HOS tumor-bearing mice following an administration with FeS2@COF-HA at pre-set time. (b) Fluorescence images of the major organs and tumors at 6 h following corresponding treatments ex vivo. (c) Fluorescence images of the tumor tissues harvested from the tumor-bearing at 6 h post injection. (d, e) Thermal images and corresponding temperature curve of tumor-bearing mice injected with PBS or FeS2@COF-HA under 808 nm laser irradiation (1.0 W/cm2) for 10 min
In vivo anti-tumor effectiveness evaluation and M1 macrophage polarization
Inspired by the above results, the therapeutic results of subcutaneous MNNG/HOS induced tumor model were shown in Fig. 7a. When the tumor volume grew to approximately 100 mm3, the mice were randomly divided into the following six group: (1) control, (2) laser, (3) AIPH + laser, (4) FeS2@COF-HA, (5) FeS2@COF-HA + laser, (6) FeS2@COF-HA/AIPH + laser. As displayed in Fig. 7c-e, it is noted that the rapid tumor growth was found in control group and littler therapeutic effect for laser and AIPH + laser group. However, both FeS2@COF-HA and FeS2@COF-HA + laser treatment exhibited certain inhibitory effect, owing to the ability of CDT and PTT/CDT to induce tumor cell death. More importantly, the mice treated with FeS2@COF-HA/AIPH + laser showed minimal tumor volume, arising from that the synergistic effects of PTT/CDT/TDT and immunotherapy. In addition, there was no significant weight fluctuations of the mice during the whole treatment period (Fig. S10), suggesting that the as-synthesized nanocomposites had no systemic side effects.
Following 14 days of treatment, Hematoxylin and eosin (H&E) and TdT-mediated dUTP Nick-End Labeling (TUNEL) staining were further carried out to further elucidate the mechanisms of tumor apoptosis (Fig. 7f). It is found that H&E-staining images exhibited that FeS2@COF-HA/AIPH + laser treated mice suffered more severe tumor cell death than other groups. Meanwhile, TUNEL staining images revealed that tumors in FeS2@COF-HA/AIPH + laser group exerted a high level of cell apoptosis than other groups. Furthermore, Ki-67 antibody staining of tumors indicated that there was a significant suppression on tumor cell proliferation in the FeS2@COF-HA/AIPH group compared to other treatment groups. To further detect the ROS production in the tumor, ex vivo ROS staining was also conducted. Compared with other experimental groups with relatively low ROS signals, the FeS2@COF-HA/AIPH + laser group exhibited stronger red fluorescence (Fig. 7f), demonstrating much better ROS production in the tumor site. Collectively, the above results indicated that combining multimodal therapies enhanced the antitumor therapeutic efficiency.
To further validate TAM reprogramming in vivo, the tumor tissues were collected and stained with CD206 and iNOS. The immunofluorescence staining results exhibited that the iNOS expression in FeS2@COF-HA/AIPH + laser group was significantly up-regulated, while the CD206 expression decreased apparently (>Fig. S9). These results further suggested that the FeS2@COF-HA/AIPH NPs + laser could effectively activate the antitumor immune response and indeed improve the tumor immunotherapeutic efficacy.
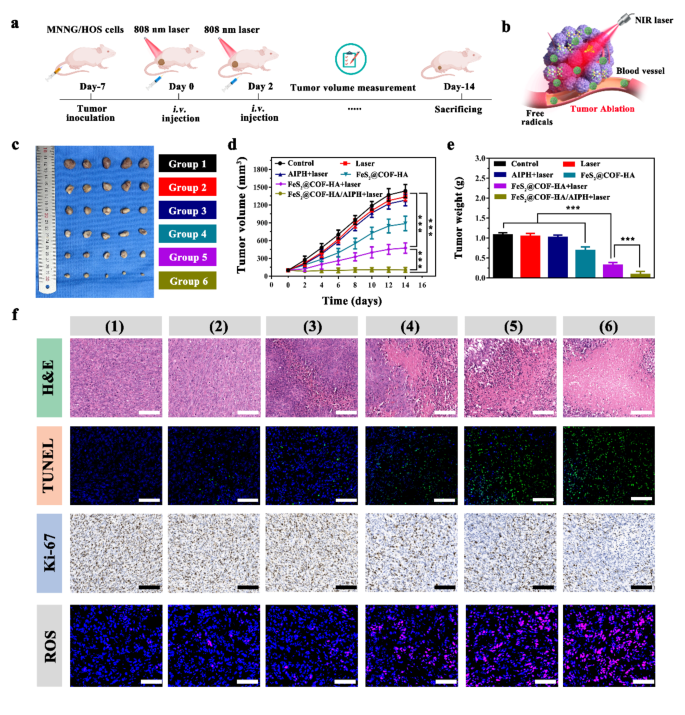
In vivo tumor therapeutic effect and mechanism analysis of as-synthesized nanoplatform. (a, b) Schematic illustration of the MNNG/HOS tumor experimental design and NIR-induced PTT/CDT/TDT combinatorial effect on tumor ablation. (c) Digital images of the tumor dissection. (d, e) Average tumor volume and tumor weight after treated with various groups (n = 5, ***P < 0.001). (f) H&E, TUNEL, Ki-67 and ROS staining images of the dissected tumor tissues in different treatment groups: (1) control, (2) laser, (3) AIPH + laser, (4) FeS2@COF-HA, (5) FeS2@COF-HA + laser, (6) FeS2@COF-HA/AIPH + laser (Scale bar = 100 μm)
Biological safety evaluation
Biocompatibility and low toxicity of the nanocomposites is highly necessary for tumor therapy. As illustrated in Fig. S11a, FeS2@COF-HA NPs exhibited a superior cytocompatibility with the high survival rate even at high concentration. In addition, hemolysis testing showed that all samples exhibited negligible hemolytic effect even exposed to a high concentration of 800 µg/mL, demonstrating the excellent hemocompatibility of as-synthesized FeS2@COF-HA NPs (Fig. S11b). At the same time, no appreciable organ damage was observed in the H&E staining of tissue slices collected form the NPs-treated mice, indicating the excellent biosafety of the as-synthesized nanoplatform (Fig. S11c). Moreover, the results of blood biochemical indexes further validated favorable biosafety of FeS2@COF-HA NPs (Fig. S12). Accordingly, the above results suggested that FeS2@COF-HA is an ideal candidate for tumor therapy owing to its outstanding biosafety.
Mechanism of FeS2@COF-HA/AIPH NPs synergistic cancer therapy analyzed by RNA-sequencing
To further figure out the potential therapeutic mechanism of FeS2@COF-HA/AIPH-mediated combined antitumor performance, the RNA-sequencing was performed (Fig. 8a). As shown in Fig. 8b, the volcano plot indicated that 767 genes were significantly up-regulated and 214 genes were significantly down-regulated. Among them, oxidative stress related genes, including GPX8, GSR, and LOX, etc. are regulated, demonstrating that the generated ROS acts an important role in tumor growth inhibition. Meanwhile, the expression of tumor necrosis factor (TNF) signaling pathway related genes (CAPSE-3, BTRAF1, and BCL3, etc.) and apoptosis related genes (FOS, CAPN2, and CASP8, etc.) was also regulated in the experimental group. In addition, the experimental group significantly upregulated the expression of immune-related genes, such as LTA, HPX, and LTF, etc. (Fig. 8c). Afterwards, Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis were further carried out to analyze the genes set enrichment. GO analysis revealed that most of the DEGs were primarily involved in tumor necrosis and immune-related pathways (Fig. 8d). KEGG analysis shows that DEGs are mainly concentrated in TNF signaling pathway, NF-kappa B signaling pathway, mitogen-activated protein kinase (MAPK) signaling pathway, and p53 signaling pathway, etc. Gene set enrichment analysis (GSEA) analysis further revelated that the experimental group increased the expression of genes were focused on the IL-17 signaling pathway, the MAPK signaling pathway, the TNF signaling pathway, and the reactive oxygen species pathway (Fig. 8e). Therefore, these results indicated that FeS2@COF-HA/AIPH + laser treatment could evoke an inflammatory storm and antitumor immune responses, thereby causing tumor cell death.
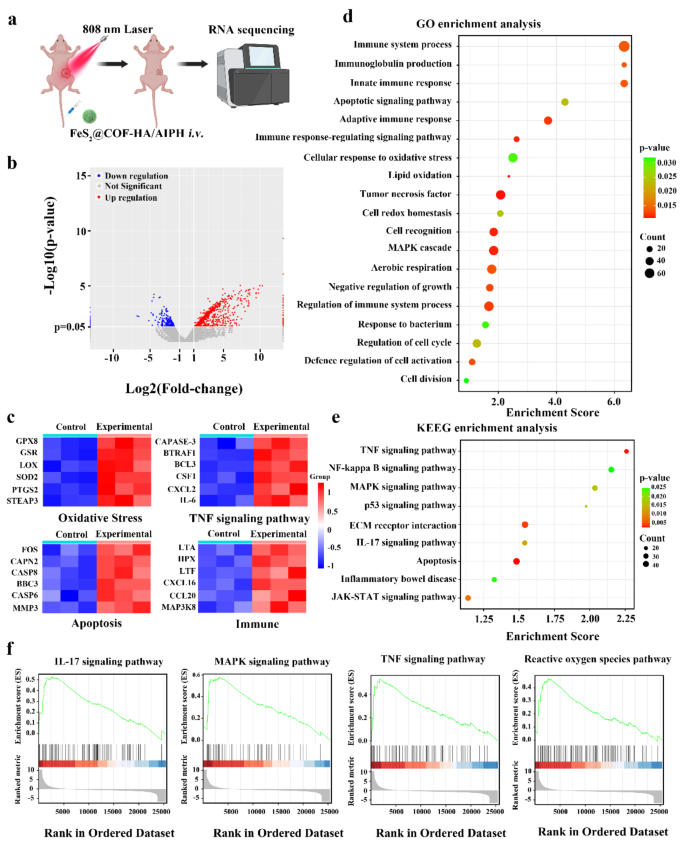
Mechanism of FeS2@COF-HA/AIPH + laser for synergistic anti-tumor therapy. (a) Schematic illustration of RNA sequencing. (b) Volcano plot of DEGs between control group and FeS2@COF-HA/AIPH + laser group. (c) Heat map of DEGs associated with oxidative stress, TNF signaling pathway apoptosis, and immune response. (d, e) GO enrichment analysis of major types of biological processes and KEEG pathway analysis based on RNA-seq after the FeS2@COF-HA/AIPH + laser treatment. (f) GSEA enrichment analysis of DEGs between control group and FeS2@COF-HA/AIPH + laser group



